Eazyplex BloodScreen GN
The eazyplex® BloodScreen GN test system is a qualitive in vitro diagnositic medical device for the detection, and reliable indentification of Gram-negative pathogens from positive flagged blood culture vials.
The following panels are available:
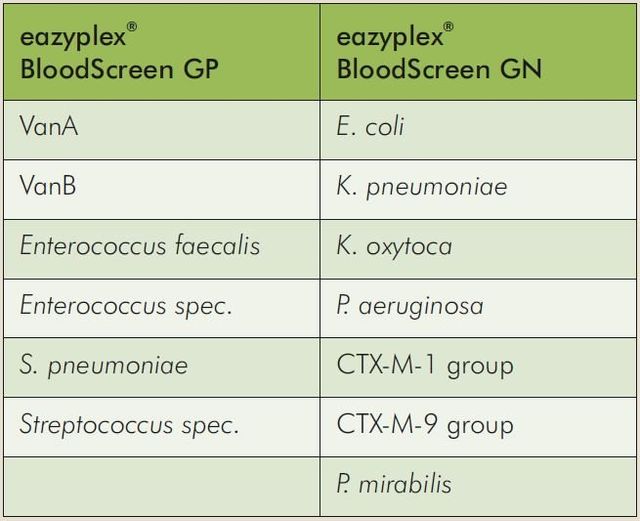
- eazyplex®BloodScreen GN, for the determination of E. coli, K. pneumoniae, K. oxytoca, P. aeruginosa, CTX-M-1 group, CTX-M-9 group, P. mirabilis.
- eazyplex®BloodScreen GP, for the determination of VanA, VanB, Enterococcus faecalis, Enterococcus spec., S. pneuomiae, Streptococcus spec.
The test can be performed at any time by qualified professional staff in a medical laboratory in order to:
- Aid in the diagnosis (providing additional information to assist in the determination or verification of a patient's clinical status, test is not the sole determinant) of all kinds of patients via testing positive blood culture vials.
In addition to 5 different Gram-negative pathogens, eazyplex® BloodScreen GN also detects the two most frequent gene groups that genetically determine an ESBL phenotype (CTX-M-1 and CTX-M-9 group).

The most common parameters are available with the eazyplex® BloodScreen Panels for the Gram-positive and Gram-negative spectrum.
The examination is carried out directly from the positive blood culture without DNA extraction,
- Hands-on-time of approximately 3 minutes.
- First signals in less than 10 minutes
- Complete test run finishing within as little as 20 minutes
- Results can be exported as PDF, CSV and adhesive label.
All signals are displayed in real-time during the amplification process!
Protective mechanisms to prevent the use of false results:
- Performance of "Inhibition control" with each sample, preventing the use of false negative tests due to the inhibition of the amplification reaction and simultaneously serves as reagent control.
- The test result is only displayed if the inhibition control is valid.
- A test strip can be processed as a negative/ contamination control when required. Achieved via the testing of RALF without the addition of sample material. In this case only the inhibition control is allowed to create a positive signal.
Specifications
- Quantity: 24
- Product Code: AM-E7676